
MEDICAL TRANSLATION
You’re looking for someone who not only speaks the language but understands the landscape, someone with both subject-matter expertise and deep familiarity with industry regulations and workflows.
That’s where I come in.
I regularly support Life Sciences companies, CROs, LSPs, and Medical Communication agencies with specialized translation and localization services across a wide range of materials, including:
Medical
Linguistic Validation
Clinical and Regulatory Content
Clinical trial documents (protocols, CRFs, ICFs, IBs, lay summaries, patient information sheets)
Study documentation (reports, pharmacological studies, SOPs)
Regulatory submissions and compliance documents (SmPCs, PILs, labelling) Study documentation (reports, pharmacological studies, SOPs).
Medical Devices
Instructions for Use (IFUs)
Safety and performance summaries
Product labeling and accompanying documentation.
Patient-Facing and Promotional Materials
Patient engagement and recruitment materials
Educational content and plain language summaries
Marketing copy, brochures, press releases.
Scientific and Digital Content
Manuscripts, abstracts, and conference posters
Training modules and internal resources
Websites and mobile apps.
Clinical Outcome Assessment (COA) measures
Clinician-Reported Outcomes (ClinRO)
Patient-Reported Outcomes (PRO)
Performance-Based Outcomes (PerfO)
Observer-Reported Outcomes (ObsRO)
Quality of Life (QoL) questionnaires
Patient diaries and symptom trackers.
These tools are essential for capturing meaningful patient and clinician input in global clinical trials. I work to make sure your instruments remain accurate, reliable, and patient-centered in every language.
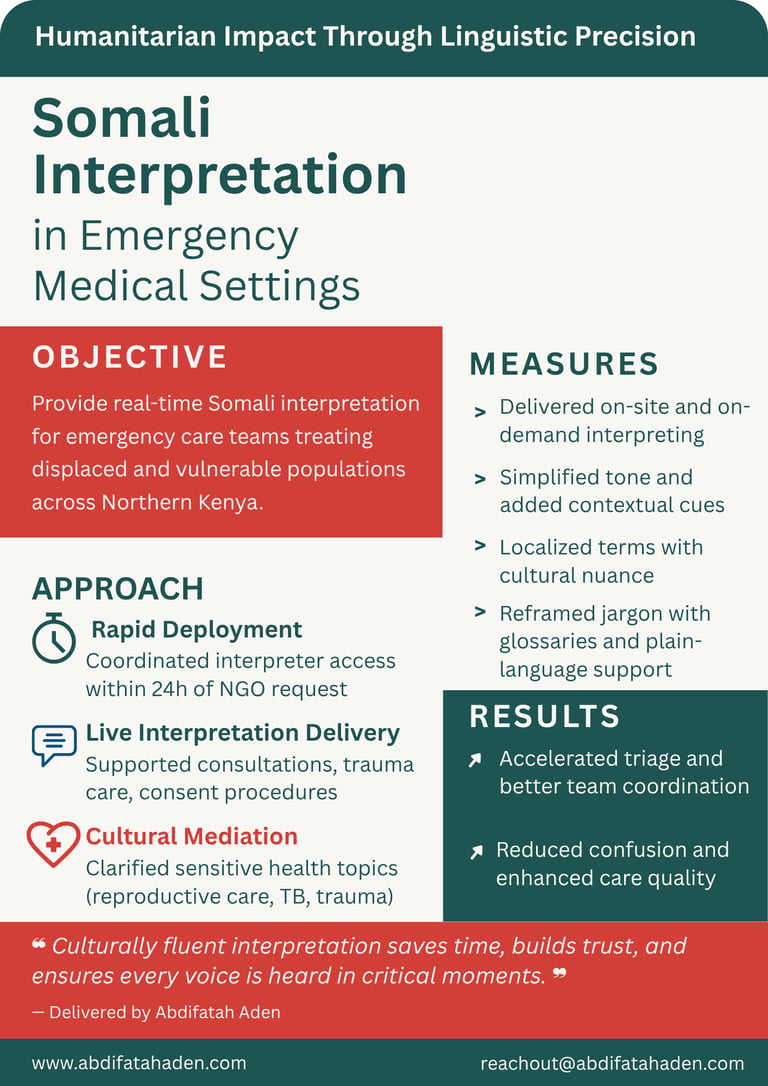
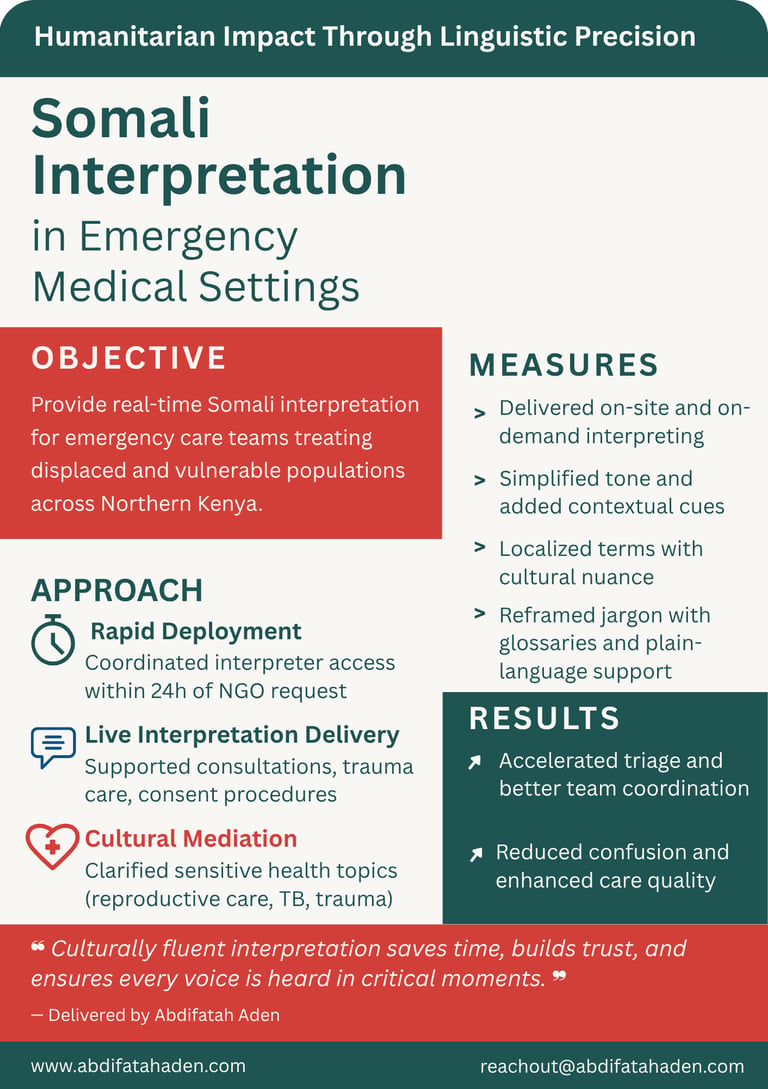
Case Studies
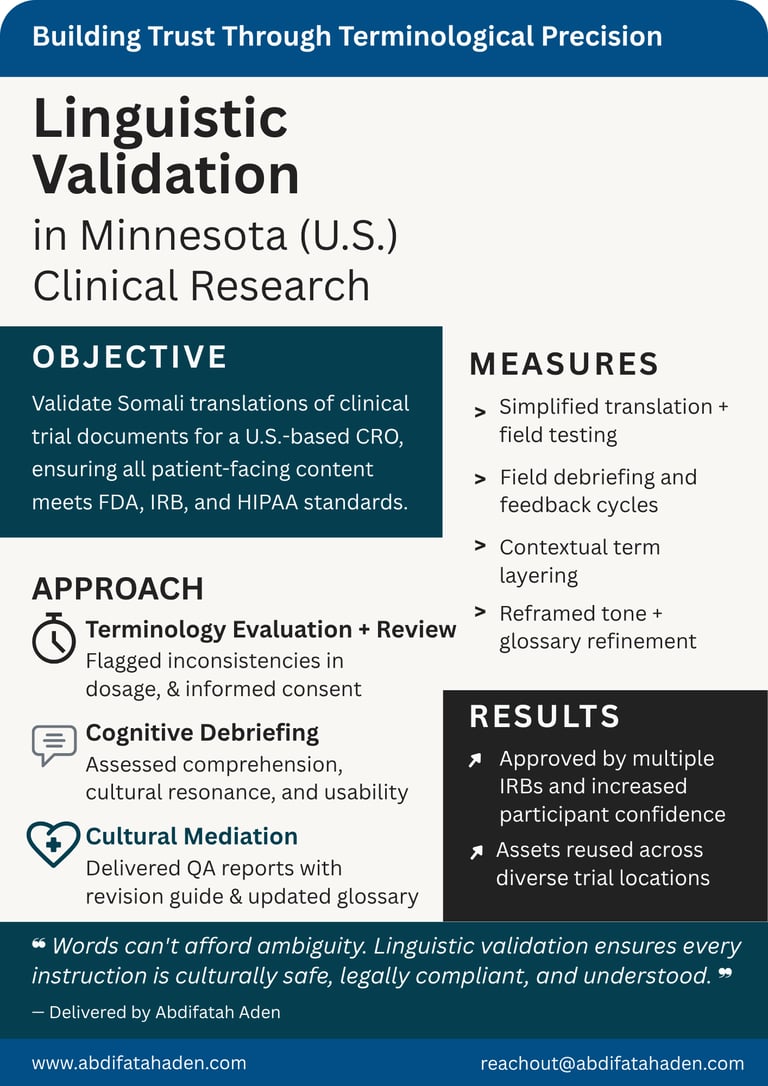
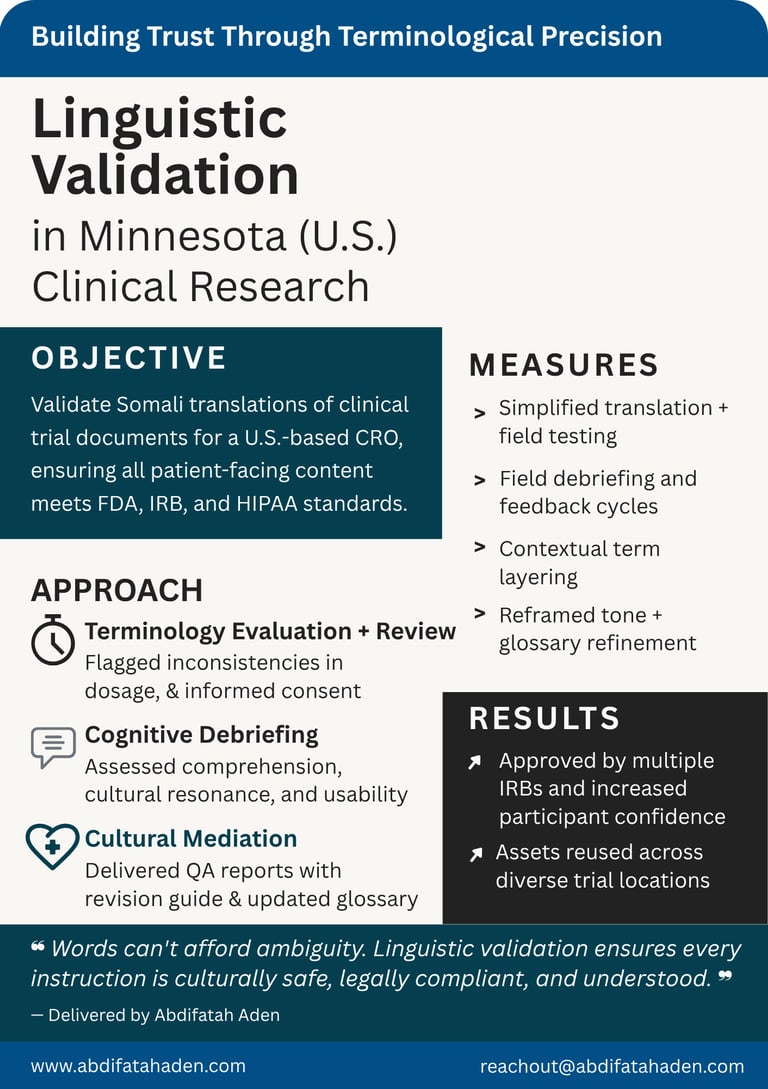
Healthcare
eLearning & Training
Patient-Facing Materials
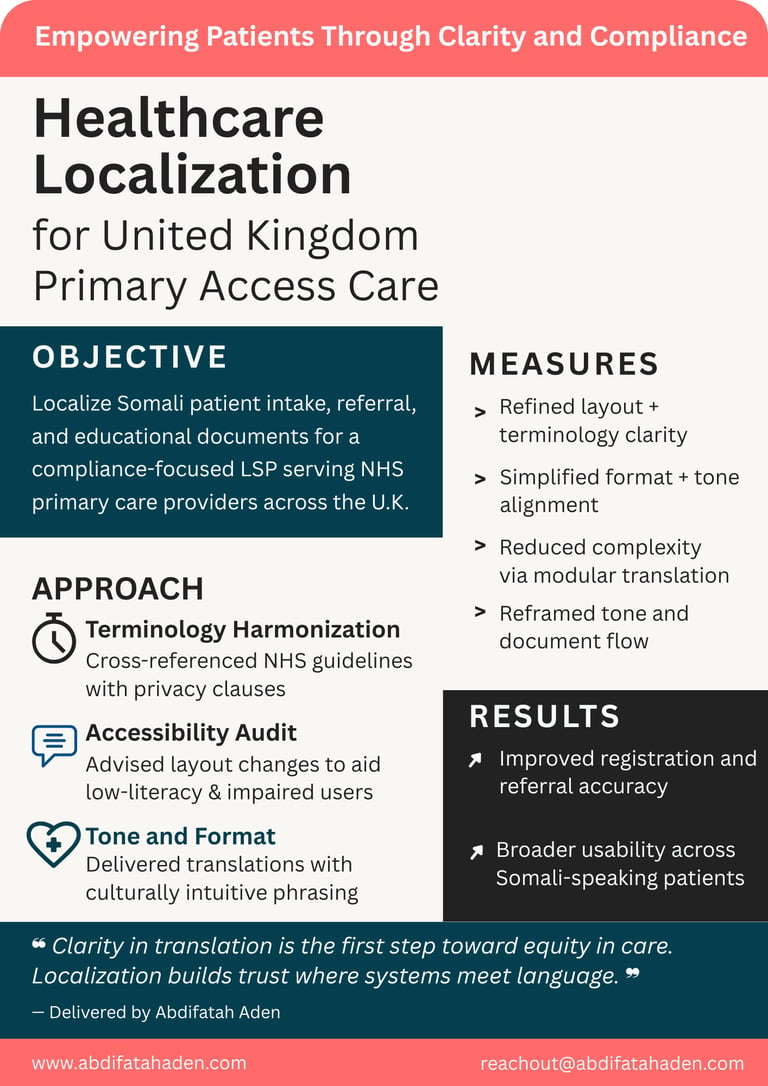
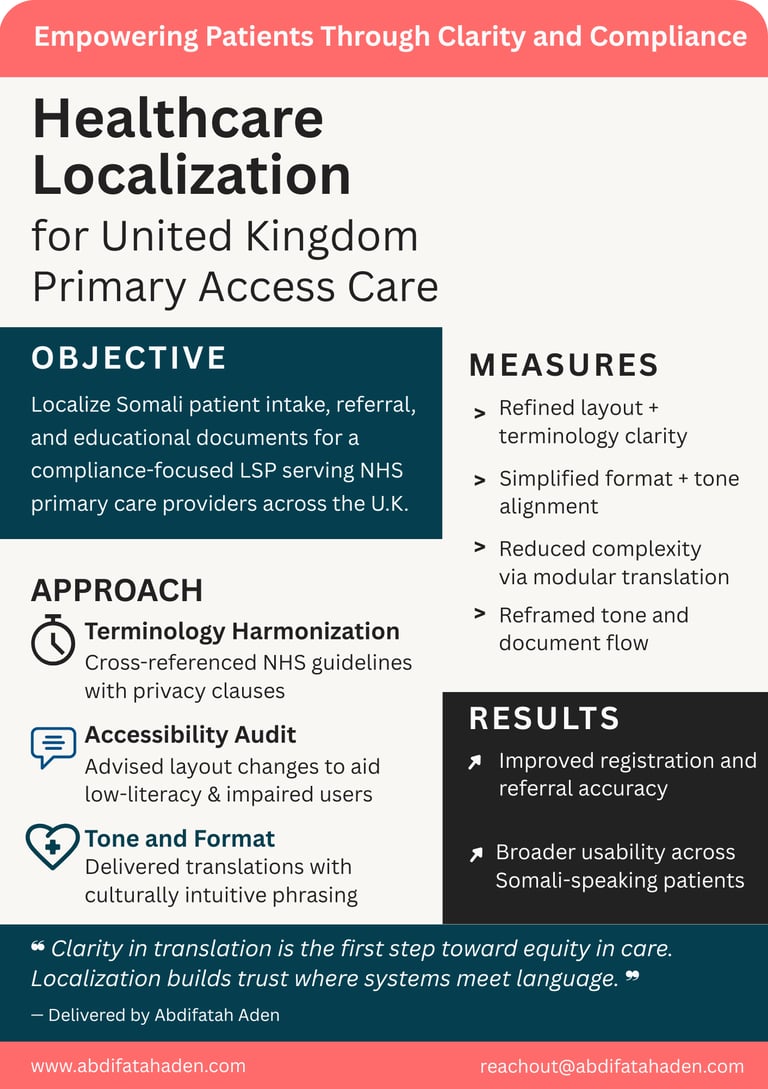
Admission and discharge forms
Patient information leaflets (PILs)
Consent forms and patient instructions
Appointment reminders and notifications
Educational brochures and plain-language content
Multilingual hospital signage and directions
Health questionnaires and feedback forms.
Clinical and Research Documentation
Regulatory submissions (e.g., EMA, FDA)
Marketing Authorization Applications (MAA, NDA, BLA)
Risk management plans (RMPs)
Periodic Safety Update Reports (PSURs)
Product labeling and packaging
Certificates of pharmaceutical products (CPPs).
Pharmacovigilance
Adverse event (AE) reports
Safety summaries and narratives
Individual Case Safety Reports (ICSRs)
Risk communication for HCPs and patients.
Patient Education and Self-Management
eLearning modules on disease awareness
Medication adherence programs
Video guides for device or treatment use
Lifestyle and condition management tools.
These materials play a vital role in delivering safe, effective, and accessible healthcare around the world. Whether it’s supporting informed patient care, ensuring regulatory compliance, or empowering learners through training, I make sure your content is clear, accurate, and culturally appropriate in every language.


Abdifatah Aden
Privacy | Terms & Conditions | Cookie Policy
© 2025. All Rights Reserved